Sustainable Hydrogen Production
The world needs hydrogen to decarbonise human activity, but current hydrogen production is based on methane, and this releases CO2. Omnagen’s SPOCC Reactor can re-use that CO2 by reacting it with methane and air at elevated temperature: once at temperature, the reactions are self-sustaining.
Large-scale hydrogen production reacts methane with steam to make hydrogen plus CO2. This is known as 'grey' hydrogen, and according to the IEA* releases 830Mt/year of CO2 into the atmosphere. This is more than the combined total emissions of the UK and France. It is not only a large contribution towards global warming, but also a wasted resource, because the chemical industry needs carbon to make its products. We propose using CO2 and natural gas as the raw materials, rather than oil and natural gas.
This one application of the SPOCC Reactor has the potential to save 2.2% of global CO2 emissions!
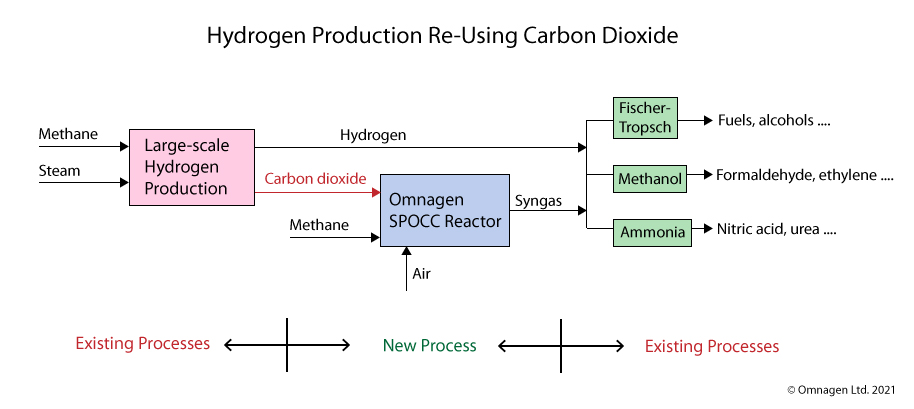
The image below shows three possible production routes using the combined raw materials of hydrogen, and syngas from the SPOCC Reactor. Each route can produce a range of chemicals, and many of those can be further converted into a vast range of other chemicals:-
- Fischer-Tropsch synthesis – producing fuels, alcohols…..
- Methanol production, then – formaldehyde, acetic acid, ethylene…..
- Ammonia production by the Haber process, then – nitric acid, urea…..
'Sky Blue' Hydrogen
There is an urgent need to make existing 'grey' hydrogen production facilities more sustainable. Some favour CCS (carbon capture and storage), where the CO2 is captured, liquified and buried; this is referred to as 'blue' hydrogen. But CO2 is a valuable source of carbon, and it makes sense to re-use it rather than bury it. We use the term 'sky blue' hydrogen to describe our method for making 'grey' hydrogen sustainable.
'Green' Hydrogen
‘Green’ hydrogen is produced by water electrolysis using renewable energy. It is energy intensive, requiring about 50kWh of energy to produce a kg of hydrogen. It also uses plenty of fresh water, requiring 9kgs of water for each kg of hydrogen produced. Sea water will not do, Therefore the scale of ‘green’ hydrogen which is possible, is dependent on supplies of both renewable energy and fresh water.
SPOCC Reactor
The Self-Powered CO2 Converter combines methane, CO2 and air at elevated temperature. Once at temperature, the reactions are self-sustaining.
Take a look to see how the technology works.
Sustainable Aviation Fuel
Anaerobic digesters convert organic matter into a mixture of methane and CO2. The SPOCC Reactor can be used as part of a route to produce sustainable aviation fuel. For an explanation.
Cement Decarbonisation
Cement production is responsible for at least 5% of global carbon dioxide emissions. Where a supply of natural gas is available, we propose capturing the CO2, adding natural gas and air, and making syngas at the site. This can be further processed to valuable chemicals.


